Cell Membrane and Transport Across the Cell Membrane Regents Review
Learning Objectives:
- Compare and contrast passive and active transport
- Describe the process of diffusion, osmosis, and facilitated diffusion
- Depict the process of the sodium/potassium pump and endo/exocytosis
- Draw the three types of endocytosis
Transport across the Jail cell Membrane
I of the great wonders of the cell membrane is its ability to regulate the concentration of substances inside the cell. These substances include ions such as Ca++, Na+, Grand+, and Cl–; nutrients including sugars, fatty acids, and amino acids; and waste products, especially carbon dioxide (CO2), which must leave the cell. The membrane's lipid bilayer structure provides the kickoff level of control. The phospholipids are tightly packed together, and the membrane has a hydrophobic interior. This structure causes the membrane to be selectively permeable. A membrane that hasselective permeability allows only substances coming together sure criteria to pass through it unaided. In the case of the prison cell membrane, only relatively small-scale, nonpolar materials tin can motility through the lipid bilayer (retrieve, the lipid tails of the membrane are nonpolar). Some examples of these are other lipids, oxygen and carbon dioxide gases, and booze. Yet, water-soluble materials—like glucose, amino acids, and electrolytes—demand some assistance to cross the membrane considering they are repelled by the hydrophobic tails of the phospholipid bilayer.
All substances that motion through the membrane exercise so by one of two general methods, which are categorized based on whether or not energy is required. Passive transport is the movement of substances across the membrane without the expenditure of cellular free energy. In contrast, active transport is the motion of substances beyond the membrane using energy from adenosine triphosphate (ATP).
Passive Ship
In social club to understandhowsubstances move passively across a cell membrane, information technology is necessary to understand concentration gradients and diffusion. A concentration gradient is the difference in concentration of a substance beyond a space. Molecules (or ions) will spread/diffuse from where they are more than full-bodied to where they are less concentrated until they are equally distributed in that space. (When molecules move in this way, they are said to move downwardly their concentration gradient.) 3 common types of passive ship include elementary diffusion, osmosis, and facilitated diffusion.
Elementary Improvidence is the movement of particles from an surface area of higher concentration to an surface area of lower concentration. A couple of common examples will aid to illustrate this concept. Imagine being inside a airtight bathroom. If a canteen of perfume were sprayed, the odor molecules would naturally diffuse from the spot where they left the canteen to all corners of the bathroom, and this diffusion would get on until no more concentration gradient remains. Another example is a spoonful of sugar placed in a cup of tea. Eventually the sugar will diffuse throughout the tea until no concentration slope remains. In both cases, if the room is warmer or the tea hotter, diffusion occurs even faster every bit the molecules are bumping into each other and spreading out faster than at libation temperatures. Having an internal body temperature around 98.six°F thus also aids in diffusion of particles within the body.
Visit this link to see diffusion and how it is propelled by the kinetic energy of molecules in solution. How does temperature affect diffusion rate, and why?
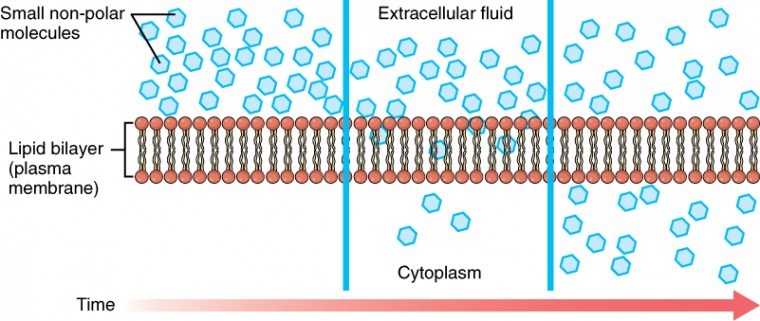
Whenever a substance exists in greater concentration on i side of a semipermeable membrane, such every bit the plasma membrane, any substance that tin can move downward its concentration gradient across the membrane will do so. Consider substances that can easily diffuse through the lipid bilayer of the prison cell membrane, such every bit the gases oxygen (O2) and CO2. Otwo generally diffuses into cells because it is more full-bodied outside of them, and COtwo typically diffuses out of cells because it is more full-bodied inside of them. Neither of these examples requires whatever energy on the part of the jail cell, and therefore they use passive send to movement across the membrane. Before moving on, y'all demand to review the gases that can diffuse across a cell membrane. Because cells apace use up oxygen during metabolism, there is typically a lower concentration of O2 inside the cell than exterior. As a result, oxygen will lengthened from the interstitial fluid directly through the lipid bilayer of the membrane and into the cytoplasm within the cell. On the other hand, considering cells produce CO2 as a byproduct of metabolism, COii concentrations rise within the cytoplasm; therefore, CO2 volition move from the jail cell through the lipid bilayer and into the interstitial fluid, where its concentration is lower. This machinery of molecules spreading from where they are more full-bodied to where they are less concentration is a grade of passive transport called simple diffusion (Figure 3.15).
Osmosis is the diffusion of water through a semipermeable membrane (Figure 3.sixteen). Water tin move freely beyond the prison cell membrane of all cells, either through poly peptide channels or past slipping between the lipid tails of the membrane itself. However, it is concentration of solutes within the h2o that determine whether or not water will be moving into the cell, out of the cell, or both.

Solutes within a solution create osmotic force per unit area, a pressure that pulls water. Osmosis occurs when at that place is an imbalance of solutes outside of a cell versus inside the cell. The more solute a solution contains, the greater the osmotic pressure that solution will have. A solution that has a higher concentration of solutes than another solution is said to be hypertonic. Water molecules tend to diffuse into a hypertonic solution because the higher osmotic pressure level pulls water (Figure 3.17). If a prison cell is placed in a hypertonic solution, the cells volition shrivel or crenate as water leaves the cell via osmosis. In contrast, a solution that has a lower concentration of solutes than some other solution is said to be hypotonic. Cells in a hypotonic solution will accept on too much water and swell, with the risk of eventually bursting, a process called lysis. A critical aspect of homeostasis in living things is to create an internal environment in which all of the body'due south cells are in an isotonic solution, an surroundings in which two solutions have the aforementioned concentration of solutes (equal osmotic pressure). When cells and their extracellular environments are isotonic, the concentration of h2o molecules is the aforementioned outside and inside the cells, and then water flows both in and out and the cells maintain their normal shape (and function). Diverse organ systems, particularly the kidneys, piece of work to maintain this homeostasis.

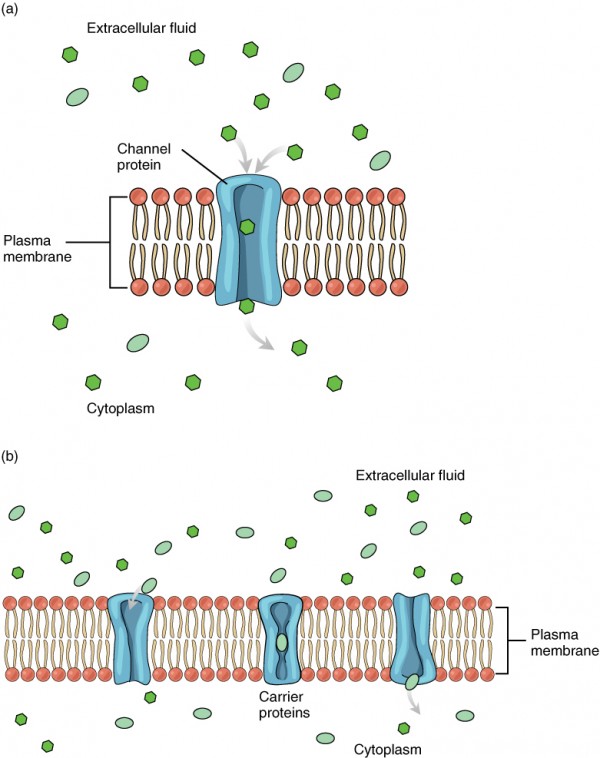
Facilitated diffusion is the diffusion process used for those substances that cannot cross the lipid bilayer due to their size and/or polarity (Effigy 3.eighteen). A common example of facilitated diffusion is the movement of glucose into the cell, where it is used to brand ATP. Although glucose can exist more concentrated exterior of a prison cell, it cannot cross the lipid bilayer via unproblematic improvidence considering it is both large and polar. To resolve this, a specialized carrier protein called the glucose transporter will transfer glucose molecules into the jail cell to facilitate its inward diffusion. In that location are many other solutes that must undergo facilitated improvidence to movement into a prison cell, such equally amino acids, or to move out of a cell, such equally wastes. Because facilitated diffusion is a passive process, it does not require energy expenditure by the prison cell.
Agile Ship
For all of the transport methods described to a higher place, the prison cell expends no energy. Membrane proteins that aid in the passive transport of substances practice so without the use of ATP. During agile ship, ATP is required to movement a substance across a membrane, often with the assist of protein carriers, and usually against its concentration gradient. One of the well-nigh mutual types of active ship involves proteins that serve as pumps. The word "pump" probably conjures up thoughts of using energy to pump up the tire of a cycle or a basketball game. Similarly, energy from ATP is required for these membrane proteins to transport substances—molecules or ions—beyond the membrane, commonly against their concentration gradients (from an area of low concentration to an area of high concentration). The sodium-potassium pump , which is also called N+/K+ ATPase, transports sodium out of a cell while moving potassium into the cell. The Na+/K+ pump is an important ion pump found in the membranes of many types of cells. These pumps are specially abundant in nerve cells, which are constantly pumping out sodium ions and pulling in potassium ions to maintain an electrical gradient across their prison cell membranes. Anelectrical slope is a divergence in electrical accuse across a space. In the case of nerve cells, for instance, the electric gradient exists between the inside and exterior of the cell, with the inside being negatively-charged (at around -70 mV) relative to the outside. The negative electrical slope is maintained because each Na+/Yard+ pump moves three Na+ ions out of the cell and two K+ ions into the prison cell for each ATP molecule that is used (Effigy 3.19). This process is and then of import for nervus cells that it accounts for the majority of their ATP usage.

Other forms of active send do non involve membrane carriers. Endocytosis (bringing "into the cell") is the process of a cell ingesting material by enveloping information technology in a portion of its prison cell membrane, and then pinching off that portion of membrane (Figure three.twenty). In one case pinched off, the portion of membrane and its contents becomes an independent, intracellular vesicle. Avesicle is a membranous sac—a spherical and hollow organelle divisional past a lipid bilayer membrane. Endocytosis often brings materials into the cell that must to be broken down or digested. Phagocytosis ("cell eating") is the endocytosis of large particles. Many allowed cells appoint in phagocytosis of invading pathogens. Like little Pac-men, their job is to patrol body tissues for unwanted matter, such as invading bacterial cells, phagocytize them, and digest them. In dissimilarity to phagocytosis, pinocytosis ("cell drinking") brings fluid containing dissolved substances into a cell through membrane vesicles. Phagocytosis and pinocytosis take in large portions of extracellular material, and they are typically not highly selective in the substances they bring in. Cells regulate the endocytosis of specific substances via receptor-mediated endocytosis.Receptor-mediated endocytosis is endocytosis past a portion of the jail cell membrane that contains many receptors that are specific for a sure substance. Once the surface receptors have spring sufficient amounts of the specific substance (the receptor's ligand), the prison cell will endocytose the office of the cell membrane containing the receptor-ligand complexes. Iron, a required component of hemoglobin, is endocytosed by scarlet claret cells in this mode.

In dissimilarity with endocytosis, exocytosis (taking "out of the jail cell") is the process of a jail cell exporting material using vesicular transport (Figure 3.21). Many cells manufacture substances that must be secreted, like a factory manufacturing a product for export. These substances are typically packaged into membrane-spring vesicles within the cell. When the vesicle membrane fuses with the jail cell membrane, the vesicle releases information technology contents into the interstitial fluid. The vesicle membrane then becomes role of the cell membrane. Cells of the stomach and pancreas produce and secrete digestive enzymes through exocytosis (Effigy 3.22). Endocrine cells produce and secrete hormones that are sent throughout the body, and certain immune cells produce and secrete large amounts of histamine, a chemical important for immune responses.


Source: https://courses.lumenlearning.com/nemcc-ap/chapter/3204/
0 Response to "Cell Membrane and Transport Across the Cell Membrane Regents Review"
Post a Comment